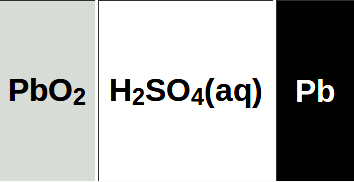|
BATTERY FORMATS
Please use our A-Z INDEX to navigate this site where page links may lead to other sites, or go HOME
|
|
GENIUS - Experimentation is at the heart of discovery and development. Man had to become civilised, with a reasonably developed society to enable men of science to experiment and invent. There is no funding mechanism for unqualified inventors. There is only funding for scientists with qualifications as academics. Yet the creativity that spawns ideas often comes from self taught engineers and experimenters.
THE FIRST BATTERY
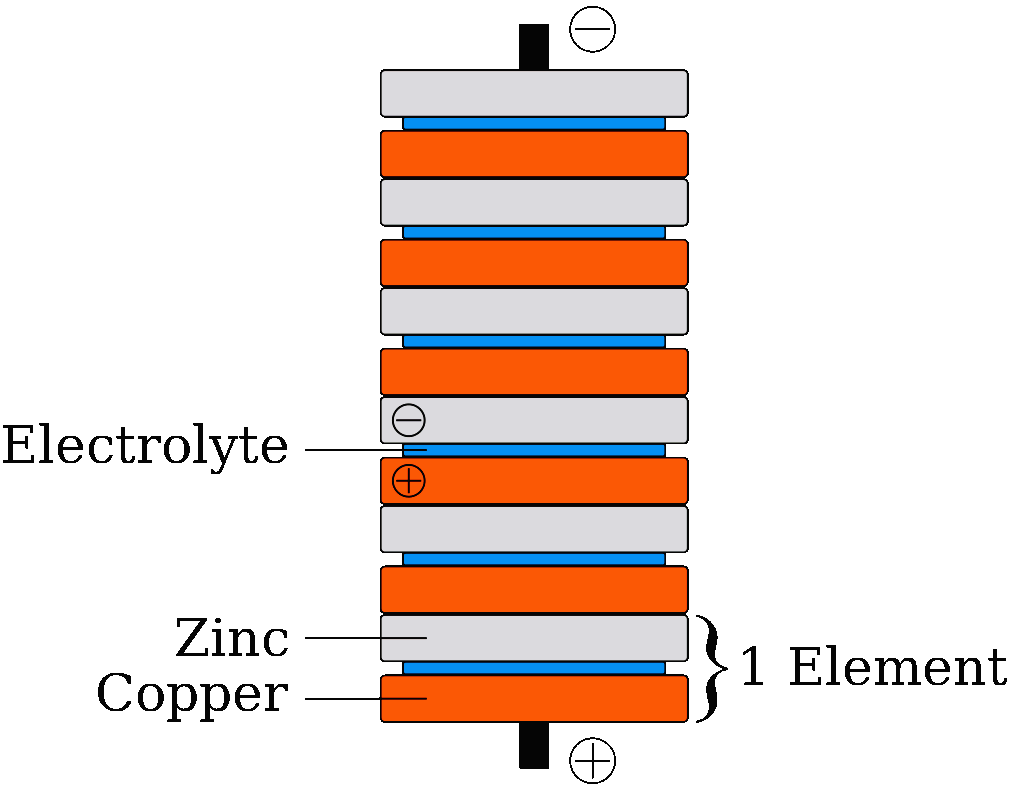
The potential for electrical energy generation using metals and chemicals was discovered by Volta, when he made his pile, a series of zinc and copper metal discs with cloth interlayers soaked in salt water to make the water conductive (an electrolyte).
The voltaic pile was the first
electric battery that could continuously provide an electric current to a circuit. It was invented by Italian physicist Alessandro Volta, who published his experiments in 1799. The voltaic pile enabled a rapid series of discoveries including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle (1800), so starting the science of electrochemistry.
PRIMARY & SECONDARY CELL BATTERIES
Batteries
fall into two categories: primary and secondary cells. Primary cell batteries are non rechargeable. They will be
exhausted after their component parts are used up (converted). They may also
be referred to as dry cell batteries, where the electrolytes are commonly
paste. They include aluminium-air batteries - a kind of fuel cell.
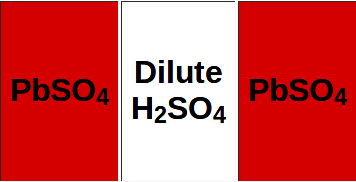
LEFT - Fully discharged: two identical lead sulfate plates and diluted sulfuric acid solution. RIGHT - Fully recharged: Lead dioxide positive plate, Lead negative plate, and concentrated, aqueous sulfuric acid solution. A weakness of batteries is leaving them discharge, causing irreversible sulfating of the plates, when the battery is useless.
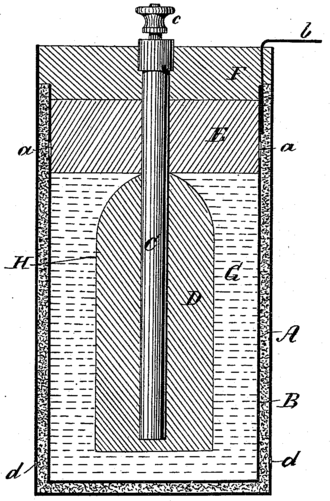
THE FIRST ACCUMULATOR (RECHARGEABLE BATTERY)
The lead–acid battery was invented in 1859 by French physicist Gaston Planté and is the earliest type of rechargeable battery. This followed the French scientist Nicolas Gautherot's observation in 1801, that wires that had been used for electrolysis experiments would themselves provide a small amount of "secondary" current after the main battery had been disconnected.
Planté's lead–acid battery was the first battery that could be recharged by passing a reverse current through it. Planté's first model consisted of two lead sheets separated by rubber strips and rolled into a spiral.
Despite having a very low energy-to-weight ratio and a low energy-to-volume ratio (energy density), the ability of the lead-acid battery to supply high surge currents means that the cells have a relatively large power-to-weight ratio. These features, and the low cost, make them ideal for use in ICE automobiles to provide the high current to turn a starter motor.
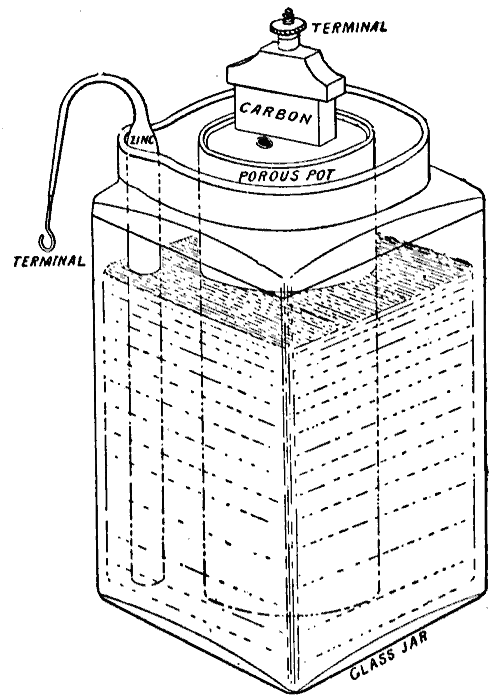
WET & DRY - The liquid electrolyte in the Leclanché cell on the left is replaced with a moist paste on the dry (drier) battery cell on the right.
THE LECLANCHÉ CELL
In 1866, Georges Leclanché invented a
(primary) battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts. This cell achieved very quick success in telegraphy,
signaling and electric bell work.
BATTERY DEVELOPMENT
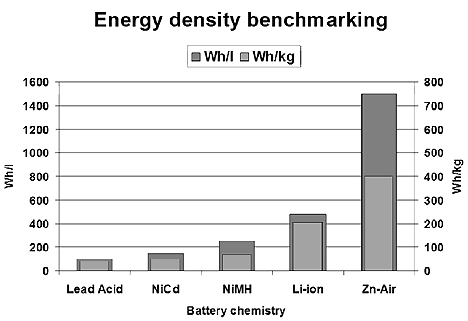
Since these early batteries, chemists have been experimenting relentlessly to improve the energy density and life cycle of cells based on silver and iron, nickel and cadmium, nickel-metal-hydride and now lithium based batteries that are the basis of most electric vehicle packs. Other metals, such as aluminum-air and zinc-air may come into the frame with more research as to commercial applications.
POWERING BUSES & TRUCKS - These EV service stations provide load leveling mobility security from renewable solar and wind. This system is the only (and the original) proposal that might refuel electric trucks, buses, SUVs, vans and of course cars.
ELECTRICITY
Electricity is the most convenient way of transmitting clean, alternative energy, from the point of origin (conversion from natural harvesting) to the end user.
Fortunately for humans, electricity is linked to magnetism, a force that can be harnessed to attract or repel, and convert a generated or stored potential difference (such as in batteries) from electrons traveling in a metal conductor, to rotational or linear movement.
This incredible property gives us electric motors and generators. We take it for granted, but it is a miracle of nature. We are living in the age of electricity where heating and lighting is electric, and computers allow us to control just about everything.
The main problem about generated electricity is storing it. This may have been solved in theory by the SMARTNET FASTCHARGE service stations for electric vehicles. The inventor has kept how this system works a secret since 1991, because he was too far ahead of his time. Even now it is risky to start divulging proprietary know-how in patent applications, because patents are expensive, with a limited shelf life.
Better Place tried something similar for cars between 2009 and 2012, but was bankrupted in 2013. Why? Mainly because he was also a tad ahead of his time. Shai Agassi's patent has expired. Fortunately, the technology is completely different.
FROM SPACE EXPLORATION TO ZERO EMISSIONS - Developed to power satellites and spacecraft, the silicon solar panel is now a cost effective way of generating clean electricity. Ideal sites for the location of solar farms is land that cannot be used for farming, such as the deserts we have created.
FUSION | BIOFUELS | GEOTHERMAL | HYDRO-ELECTRIC | SOLAR | WAVE & TIDAL | WIND
LINKS & REFERENCE
https://medium.com/@pdiwan/is-battery-swapping-a-viable-option-for-public-transportation-evs-adb4ced74ff2
ENERGY CRISIS - Some countries act as though there is no energy crisis where they have an abundance of fossil fuel reserves, but they will be unable to milk the remainder of the world with the allure of cheap fossil fuels and energy independence for renewables becomes the norm. We must help those blinded by kleptocratic policies to stop killing species and warming the planet.
Please use our A-Z INDEX to navigate this site
|
|
|
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2021. Solar Studios, BN271RF, United Kingdom.
|