|
ALUMINIUM AIR
Please use our A-Z INDEX to navigate this site where page links may lead to other sites, or see HOME
|
|
TREVOR JACKSON - Ex Royal Naval officer, inventor claims to have perfected an aluminium-air battery with a new electrolyte that is not caustic, and offers a stunning range compared to lithium batteries. These are primary cells, whereas researchers at Stanford University were working on aluminum ion cells that are rechargeable.
DAILY MAIL OCTOBER 2019 - EX NAVY OFFICE TURNS INVENTOR
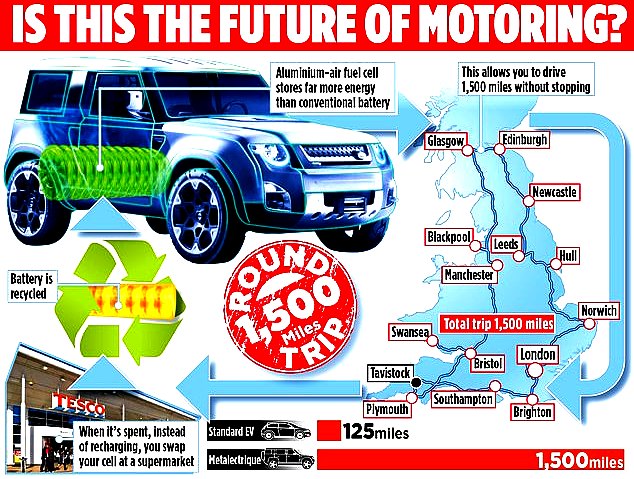
Imagine the satisfaction of driving your environmentally friendly electric car for 1,500 miles without having to stop to recharge the battery – a distance more than four times as far as the best and most expensive model currently on the road.
Under the bonnet is a revolutionary new type of battery which, unlike those used in conventional electric cars, can also power
buses, huge
lorries and even aircraft. What's more, it's far simpler and cheaper to make than the batteries currently in use in millions of electric vehicles around the world – and, unlike them, it can easily be recycled.
'It can help trigger the next industrial revolution. The advantages over traditional electric vehicle batteries are enormous,' he said.
Few will have heard of Jackson's extraordinary invention. The reason, he says, is that since he and his company Metalectrique Ltd came up with a prototype a decade ago, he has faced determined opposition from the automobile industry establishment.
It has every reason not to give ground to a competitor that may, in time, render its own technology obsolete. Car industry
sceptics claim Trevor's technology is unproven, and its benefits exaggerated.
But an independent evaluation by the Government agency UK Trade and Investment said in 2017 that it was a 'very attractive battery' based on 'well established' technology, and that it produced much more energy per kilogram than standard electric vehicle types.
He has also secured a £108,000 grant for further research from the Advanced Propulsion Centre, a partner of the Department for Business, Innovation and Skills. His technology has been validated by two French universities.
He says: 'It has been a tough battle but I'm finally making progress. From every logical standpoint, this is the way to go.'
Jackson began working on new ways of powering electric vehicles after a distinguished engineering career. He worked for Rolls-Royce in Derby, helping to design nuclear reactors, then took a commission in the Royal Navy, where he served as a lieutenant on board nuclear submarines, managing and maintaining their reactors.
Before founding his own firm in 1999, he was working for BAE Systems, where he first started looking at alternative, green ways to power vehicles. By then he and his partner, Kathryn, were married. The couple have eight children, aged 11 to 27, and live in Tavistock, on the edge of Dartmoor in Devon.
In 2001 he began to investigate the potential of a technology first developed in the 1960s. Scientists had discovered that by dipping aluminium into a chemical solution known as an electrolyte, they could trigger a reaction between the metal and air to produce electricity. At that time the method was useless for commercial batteries because the electrolyte was extremely poisonous, and caustic.
After years of experimentation at his workshop in the Cornish village of Callington, Jackson's eureka moment came when he developed a new formula for the electrolyte that was neither poisonous nor caustic.
'I've drunk it when demonstrating it to investors, so I can attest to the fact that it's harmless,' Jackson says. Another problem with the 1960s version was that it worked only with totally pure aluminium, which is very expensive.
But Jackson's electrolyte works with much lower-purity metal – including recycled drinks cans. The formula, which is top secret, is the key to his device.
Technically, it should be described as a fuel cell, not a battery. Either way, it is so light and powerful that it could now be set to revolutionise low-carbon transport, because it supplies so much energy.
Jackson gave me a demonstration. He cut off the top of a can of Coke, drained it, filled it with the electrolyte, and clipped electrodes to it, powering a small propeller. 'The energy in this will keep the propeller spinning for a month,' he said. 'You can see what this technology could do in a vehicle if you scale it up.' Following last week's deal with Austin, that is exactly what is about to happen. Three immediate projects are about to go into production.
The first is to manufacture for the Asian market some 'tuk-tuks' – the three-wheeler taxis used by the Duke and Duchess of Cambridge last week during their Royal visit to Pakistan. The second is to make electric bikes, which will be cheaper and run for much longer than those made by rivals.
Finally, and most importantly, the firm is to produce kits to convert ordinary petrol and diesel cars into hybrids, by fitting them with aluminium-air cells and electric motors on the rear wheels.
A driver will be able to choose whether to run the car on fossil fuel or electricity. The cost of each conversion, Jackson says, will be about £3,500, and they will be available early next year. This, he adds, will be the stepping-stone to a full-blown electric vehicle powered by aluminium-air fuel cells.
The car industry has already poured massive investment into a very different type of battery, lithium-ion.
Also found in devices such as computers and mobile phones, lithium-ion batteries are rechargeable. Almost every electric vehicle on the road uses them. But they have big drawbacks. As well as lithium, they contain rare, poisonous substances such as cobalt. They can explode or catch fire, as seen with the spate of incidents that forced Samsung to recall tens of thousands of Galaxy Note 7 phones in 2016. With repeated charging, car-sized models eventually become spent. Recycling them to recover the cobalt and lithium is extremely expensive – about five times as much as the cost of disposing them and starting from scratch.
Aluminium, on the other hand, is the planet's most abundant metal. Many of the factories that refine it from ore or recycled junk are powered by green, renewable energy, such as hydro-electric dams. And once an aluminium-air fuel cell is spent, it can be recycled very cheaply. According to Jackson, the cost of recycling means the running costs of an aluminium-air powered car would work out at 7p per mile. The cost of a small hatchback's petrol comes to around 12p per mile. More important, lithium-ion batteries are heavy.
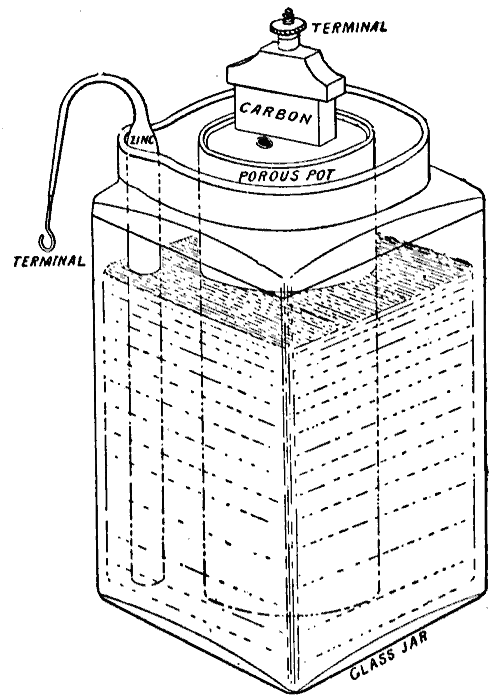
WET & DRY - The liquid electrolyte in the Leclanché cell on the left is replaced with a moist paste on the dry (drier) battery cell on the right.
Accredited tests have shown that, weight for weight, Jackson's fuel cell produces nine times as much energy as lithium-ion: nine times as many kilowatt hours of electricity per kilogram. The luxury electric car maker Tesla says its model S has a range of 370 miles from one charge. Jackson says that if you drove the same car with an aluminium-air cell that weighed the same as the lithium-ion battery, the range would be 2,700 miles. Aluminium-air cells also take up less space.
Jackson claims that if the Tesla were fitted with an aluminium-air fuel cell that was the same size as its current battery, it could run non-stop for 1,500 miles – almost enough to get from Land's End to John O'Groats and back again. An average British family – whose car will travel 7,900 miles annually – would need to change their fuel cell only a handful of times each year
He says: 'You could easily stack numerous cells in this type of vehicle – after all, getting rid of their diesel fuel tanks will give you plenty of space.' Jackson adds that aluminium-air cells could also be used in aircraft. 'We are in discussions with two aircraft manufacturers. It's not going to be suitable for jets. But it would work in propeller planes, and be suitable for short-haul passenger and cargo flights.' Meanwhile, the raw cost of a new aluminium-air cell is much lower.
In a Tesla, Jackson says, the battery costs about £30,000. An aluminium-air fuel cell that would power the same car for longer would cost just £5,000.
Drivers with cars that depend on lithium-ion have to charge their batteries from the mains when they are spent – a process that takes a long time, often overnight. But when an aluminium-air cell became exhausted, the driver would simply exchange it for a new one.
Instead of a vast network of charging points, all that is necessary are stores where cells can be swapped, just as people already swap propane gas bottles.
Swapping a battery, says Jackson, takes about 90 seconds.
He and Corcoran say they are in 'advanced discussions' with two major supermarket chains to provide this facility.
Ironically, Jackson's story so far bears more than a passing resemblance to Dyson's. Dyson developed his bagless vacuum cleaner at a workshop at his home, supported by his wife. And just as Jackson has had to battle the vested interests of the big motor companies, it took Dyson ten years to break through commercially, because no distributor or existing manufacturer was prepared to upset the lucrative market for dust bags.
'Everyone knows that if we are really going to hit the Government's target of net zero
greenhouse gas emissions by 2050, the hardest nut to crack is transport,' Jackson says. 'We're just not going to do that with lithium-ion. Apart from anything else, it's no use for trucks, which burn vast amounts of fossil fuel.
NON RECHARGEABLE PRIMARY CELLS
Aluminium–air batteries (Al–air batteries) produce electricity from the reaction of oxygen in the air with aluminium. They have one of the highest energy densities of all batteries, but they are not widely used because of problems with high anode cost and byproduct removal when using traditional electrolytes. This has restricted their use to mainly military applications. However, an electric vehicle with aluminium batteries has the potential for up to eight times the range of a lithium-ion battery with a significantly lower total weight. Clearly then, this is technology that should be investigated for future potential.
Aluminium–air batteries are primary cells, i.e., non-rechargeable. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water-based electrolyte to form hydrated aluminium oxide, the battery will no longer produce electricity. However, it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. Such recycling would be essential if aluminium–air batteries are to be widely adopted.
"The Al/air battery system can generate enough energy and power for driving ranges and acceleration similar to gasoline powered cars...the cost of aluminium as an anode can be as low as US$ 1.1/kg as long as the reaction product is recycled. The total fuel efficiency during the cycle process in Al/air electric vehicles (EVs) can be 15% (present stage) or 20% (projected), comparable to that of internal combustion engine vehicles (ICEs) (13%). The design battery energy density is 1300 Wh/kg (present) or 2000 Wh/kg (projected). The cost of battery system chosen to evaluate is US$ 30/kW (present) or US$ 29/kW (projected). Al/air EVs life-cycle analysis was conducted and compared to lead/acid and nickel metal hydride (NiMH) EVs. Only the Al/air EVs can be projected to have a travel range comparable to ICEs. From this analysis, Al/air EVs are the most promising candidates compared to ICEs in terms of travel range, purchase price, fuel cost, and life-cycle cost."
Technical problems remain to be solved to make Al–air batteries suitable for electric vehicles. Anodes made of pure aluminium are corroded by the electrolyte, so the aluminium is usually alloyed with tin or other elements. The hydrated alumina that is created by the cell reaction forms a gel-like substance at the anode and reduces the electricity output. This is an issue being addressed in the development work on Al–air cells. For example, additives that form the alumina as a powder rather than a gel have been developed.
STANFORD UNIVERSITY APRIL 2015 - RECHARGEABLE ALUMINIUM ION BATTERIES
Stanford University scientists have invented the first high-performance aluminum battery that’s fast-charging, long-lasting and inexpensive. Researchers say the new technology offers a safe alternative to many commercial batteries in wide use today.
“We have developed a rechargeable aluminum battery that may replace existing storage devices, such as alkaline batteries, which are bad for the environment, and lithium-ion batteries, which occasionally burst into flames,” said Hongjie Dai, a professor of chemistry at Stanford. “Our new battery won’t catch fire, even if you drill through it.”
Dai and his colleagues describe their novel aluminum-ion battery in “An ultrafast rechargeable aluminum-ion battery,” which will be published in the April 6 advance online edition of the journal Nature.
Aluminum has long been an attractive material for batteries, mainly because of its low cost, low flammability and high-charge storage capacity. For decades, researchers have tried unsuccessfully to develop a commercially viable aluminum-ion battery. A key challenge has been finding materials capable of producing sufficient voltage after repeated cycles of charging and discharging.
Graphite cathode
An aluminum-ion battery consists of two electrodes: a negatively charged anode made of aluminum and a positively charged cathode.
“People have tried different kinds of materials for the cathode,” Dai said. “We accidentally discovered that a simple solution is to use graphite, which is basically carbon. In our study, we identified a few types of graphite material that give us very good performance.”
For the experimental battery, the Stanford team placed the aluminum anode and graphite cathode, along with an ionic liquid electrolyte, inside a flexible polymer- coated pouch.
“The electrolyte is basically a salt that’s liquid at room temperature, so it’s very safe,” said Stanford graduate student Ming Gong, co-lead author of the Nature study.
Aluminum batteries are safer than conventional lithium-ion batteries used in millions of laptops and cell phones today, Dai added. “Lithium-ion batteries can be a fire hazard,” he said.
As an example, he pointed to recent decisions by United and Delta airlines to ban bulk lithium-battery shipments on passenger planes.
“In our study, we have videos showing that you can drill through the aluminum battery pouch, and it will continue working for a while longer without catching fire,” Dai said. “But lithium batteries can go off in an unpredictable manner – in the air, the car or in your pocket. Besides safety, we have achieved major breakthroughs in aluminum battery performance.”
One example is ultra-fast charging. Smartphone owners know that it can take hours to charge a lithium-ion battery. But the Stanford team reported “unprecedented charging times” of down to one minute with the aluminum prototype.
Durability is another important factor. Aluminum batteries developed at other laboratories usually died after just 100 charge-discharge cycles. But the Stanford battery was able to withstand more than 7,500 cycles without any loss of capacity. “This was the first time an ultra-fast aluminum-ion battery was constructed with stability over thousands of cycles,” the authors wrote.
By comparison, a typical lithium-ion battery lasts about 1,000 cycles.
“Another feature of the aluminum battery is flexibility,” Gong said. “You can bend it and fold it, so it has the potential for use in flexible electronic devices. Aluminum is also a cheaper metal than lithium.”
In addition to small electronic devices, aluminum batteries could be used to store renewable energy on the electrical grid, Dai said.
“The grid needs a battery with a long cycle life that can rapidly store and release energy,” he explained. “Our latest unpublished data suggest that an aluminum battery can be recharged tens of thousands of times. It’s hard to imagine building a huge lithium-ion battery for grid storage.”
Aluminum-ion technology also offers an environmentally friendly alternative to disposable alkaline batteries, Dai said.
“Millions of consumers use 1.5-volt AA and AAA batteries,” he said. “Our rechargeable aluminum battery generates about two volts of electricity. That’s higher than anyone has achieved with aluminum.”
“Our battery produces about half the voltage of a typical lithium battery,” he said. “But improving the cathode material could eventually increase the voltage and energy density. Otherwise, our battery has everything else you’d dream that a battery should have: inexpensive electrodes, good safety, high-speed charging, flexibility and long cycle life. I see this as a new battery in its early days. It’s quite exciting.”
Other co-lead authors of the study affiliated with Stanford are visiting scientists Mengchang Lin from the Taiwan Industrial Technology Research Institute, Bingan Lu from Hunan University, and postdoctoral scholar Yingpeng Wu. Other authors are Di-Yan Wang, Mingyun Guan, Michael Angell, Changxin Chen and Jiang Yang from Stanford; and Bing-Joe Hwang from National Taiwan University of Science and Technology.
Principal support for the research was provided by the U.S. Department of Energy, the Taiwan Industrial Technology Research Institute, the Stanford Global Climate and Energy Project, the Stanford Precourt Institute for Energy and the Taiwan Ministry of Education
Hongjie Dai, Department of Chemistry: (650) 723-4518, hdai@stanford.edu
BATTERY DEVELOPMENT
Since the early batteries, chemists have been experimenting relentlessly to improve the energy density and life cycle of cells based on silver and iron, nickel and cadmium, nickel-metal-hydride and now lithium based batteries that are the basis of most electric vehicle packs. Zinc and aluminum are less costly metals showing promise as primary and even secondary cells.
POWERING BUSES & TRUCKS - These EV service stations provide load leveling mobility security from renewable solar and wind. This system is the only (and the original) proposal that might refuel electric trucks, buses, SUVs, vans and of course cars.
ELECTRICITY
Electricity is the most convenient way of transmitting clean, alternative energy, from the point of origin (conversion from natural harvesting) to the end user.
Fortunately for humans, electricity is linked to magnetism, a force that can be harnessed to attract or repel, and convert a generated or stored potential difference (such as in batteries) from electrons traveling in a metal conductor, to rotational or linear movement.
This incredible property gives us electric motors and generators. We take it for granted, but it is a miracle of nature. We are living in the age of electricity where heating and lighting is electric, and computers allow us to control just about everything.
The main problem about generated electricity is storing it. This may have been solved in theory by the SMARTNET FASTCHARGE service stations for electric vehicles. The inventor has kept how this system works a secret since 1991, because he was too far ahead of his time. Even now it is risky to start divulging proprietary know-how in patent applications, because patents are expensive, with a limited shelf life of only 20 years, where trademarks may be renewed indefinitely and copyright lasts for 50 years after the death of the author.
FUSION | BIOFUELS | GEOTHERMAL | HYDRO-ELECTRIC | SOLAR | WAVE & TIDAL | WIND
LINKS & REFERENCE
https://news.stanford.edu/2015/04/06/aluminum-ion-battery-033115/ https://www.southampton.ac.uk/engineering/research/projects/aluminium_air_battery.page http://aluminiumair.co.uk/ https://www.dailymail.co.uk/news/article-7592485/Father-eight-invents-electric-car-battery-drivers-1-500-miles-without-charging-it.html
ENERGY CRISIS - Some countries act as though there is no energy crisis where they have an abundance of fossil fuel reserves, but they will be unable to milk the remainder of the world with the allure of cheap fossil fuels and energy independence for renewables becomes the norm. We must help those blinded by kleptocratic policies to stop killing species and warming the planet.
Please use our A-Z INDEX to navigate this site
|
|
|
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2021. Solar Studios, BN271RF, United Kingdom.
|