|
HYDROGEN HYDRIDES
Please use our A-Z
INDEX to navigate this
site where page links may lead to other sites, or see
HOME
COMPRESSED
GAS - ECONOMY - FUEL
CELLS - FUSION
- HYDRIDES
- LIQUID GAS
|
||
CHALLENGES - At present, the biggest challenge for the implementation of this technology in automotive and shipping applications is the complexity of hydrogen storage. Gas hydrogen at normal pressure and temperature conditions has a lot of energy per Kg but this is not the case of its volumetric base. To reduce its volume, it has to be compressed and stored at around 700 bar. To contain the gas at this pressure, the tank needs to have walls so thick that its weight is heavier than the gas inside. In these conditions, the contained energy per tank Kg to hydrogen ratio is drastically lower. Other alternatives for hydrogen storage are the liquid hydrogen tanks (−252,87 °C) and the storage in solid oxides. The first alternative requires high gas liquefaction costs and highly thermal isolating tanks. Also, some metal hydrides can store hydrogen in their crystal lattice and then release it at high temperatures. This technology is very promising although its current efficiency is very low.
Fuel cell-powered cars and ships have been hailed as the answer not only to eliminating our reliance on diminishing fuel supplies but also to reducing CO2 emissions from vehicles. The portable and safe storage of hydrogen will be fundamental to the exploitation of fuel cells for transport.
There is currently immense interest in hydrogen storage by industry, and international governments, and consequently many targets for hydrogen storage systems have been set up. The US Government together with the US car industry, for example, have set a target for such systems to provide at least 300 miles of travel without adding significantly to the weight or volume of a car.
To travel this distance a PEM fuel cell-powered car would require 106,000 litres of gaseous hydrogen (at
NTP). In contrast, a petrol-driven car requires 54 litres of fuel for the same journey.
At this pressure the density of hydrogen is 36kg m-3, so to store enough hydrogen for a 300-mile journey would require a 25 litre cylinder. While this is a reasonable volume, the cylinder would have to withstand very high pressures and therefore would be very heavy, which would present a problem for cars and vehicles generally. Secondly, the energy required to compress the gas represents nearly 10 per cent of the energy stored in the hydrogen.
Finally, the safety implications of every car carrying a high-pressure hydrogen cylinder are considerable. It would be like driving to work in a bomb waiting to detonate via human error or manufacturing defect.
CSIRO - Hydrogen-powered cars also have more distance range – about 800 km compared to between 160-500 km for electric cars. And if the hydrogen is derived from renewable energy sources, hydrogen-powered cars are emissions free.
However costs can be prohibitive to wider uptake, as most retail for about $80,000. Dolan said fuel for these hydrogen-powered cars would cost about $15/kg, with a tank holding about 5 kg of hydrogen.
Two cars powered by ultra-high purity hydrogen derived from ammonia were successfully tested in Brisbane, Australia. This development is the culmination of nearly a decade’s worth of work on membrane technology by CSIRO researchers. The membrane is a modular unit that can be installed at a refuelling station. It derives ultra-high purity hydrogen from ammonia, while blocking all other gases.
Why bother? Why not use battery cars with solar panels attached as an integral part of the bodywork. Australia was home to the Darwin to Adelaide solar car races that were so fast they had to raise the speed limit. With so much sunshine to tap into, it makes little sense complicating electric vehicles, other than constantly seeking more sustainable solutions.
METAL HYDRIDES - CHEMISORPTION
The storage of hydrogen in a metal hydride involves the formation of a M-H bond, where M is the metal. In the simplest case the general reaction scheme is:
The hydrogen comes into contact with the storage material (M). The hydrogen molecule is then split into hydrogen atoms, which can go on to react with the metal to form the metal hydride, or recombine to reform hydrogen. The conditions required to form the metal hydride depend on the thermodynamics of the system, and the ability of the metal to split molecular hydrogen into the atomic species.
Thermodynamically the reaction has been improved by adding nickel - the hydride's enthalpy of formation has dropped from -77.5kJ mol-1 to -64.5kJ mol-1.11 Nickel is extremely good at dissociating hydrogen into atoms whereas magnesium is very poor. So adding nickel to the system has been very favourable, the temperature of desorption for the Mg2 NiH4 is >80K, less than that of MgH2. Unfortunately the nickel increases the weight of the alloy, and therefore reduces the weight per cent hydrogen that is stored from 7.4 weight per cent (in MgH2) down to 4 weight per cent.
There is, therefore, a fine balance between capacity and absorption performance that must be maintained in our search for a new storage material. Magnesium hydride and magnesium nickel hydride are examples of interstitial hydrides. The hydrogen is absorbed into the metallic crystal structure and stored at sites within the lattice in a process that tends to be reversible.
Currently the only hydrides which are capable of achieving the 9 wt% gravimetric goal for 2015 are limited to lithium, boron and aluminium based compounds; at least one of the second-row elements or Al must be added. Research is being done to determine new compounds which can be used to meet these requirements.
A French company McPhy Energy is developing the first industrial product, based on magnesium hydride, already sold to some major clients such as Iwatani and ENEL.
NANO TECHNOLOGY
Metal hydrides have proven to be a good alternative for hydrogen storage systems because of their favorable properties such as high volumetric and gravimetric density.
However, more research is necessary to satisfy the United States Department of Energy’s requirements for storage capacity, kinetics, cyclability, cost, and release temperature. Nano-metal hydrides possess a number of properties that make them even better candidates for future hydrogen storage systems compared to their bulk equivalents.
At the nanoscale, structural and chemical properties (such as particle size and sorption site density) show a significant improvement in properties such as sorption kinetics, hydrogen diffusion or hydrogen release temperature. However, the downsides of nanoscale materials include poor heat transfer and total sorption capacity.
The Key Laboratory of Advanced Materials Technology of Liaoning, China, studied the difference in the reaction kinetics between bulk MgH2 and nanoparticles of this compound. They observed that the amount of hydrogen released by the 30 nm particle was 5wt%, 10 times greater than the bulk material (0.5wt%) over the same period of time (1 hour) and at the same temperature (300ºC).
However, a 5 wt% capacity is still not sufficient for large scale applications. Palladium (Pd) proved to be an effective alternative with favorable kinetics, but its high cost suggests favorability in using more affordable metals such as vanadium (V). A MgH2-5 wt% V showed fast adsorption kinetics in much larger quantities than Pd. A mixture of titanium and iron was also successfully used, despite these two metals being ineffective if used separately.
UTOPIAN
The allure of the hydrogen economy is plain, splitting plain ordinary water using electrolysis to obtain oxygen and hydrogen gas is like a dream come true, especially if we can generate free electricity using solar cells and wind turbines to split the water.
Then the hydrogen is free right. But is the electricity free?
No, not really.
There is a cost, including the cost of manufacturing the solar panels or wind turbines and the transmission line installation and maintenance.
Where there is a cost, then we have to consider payback time and working life. If we can use most of the solar and wind energy directly to power vehicles, we make the best of the working life of our energy harvesting apparatus. And that means reduced greenhouse gases, so a reduced carbon footprint for the human race in an anthropogenic fight against climate change.
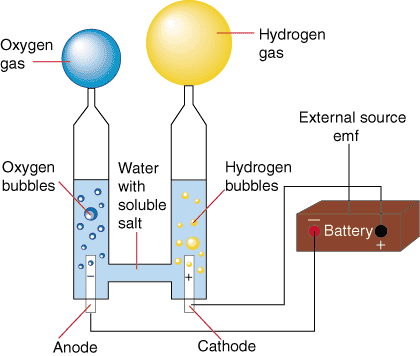
BACK TO BASICS - ELECTROLYSIS
A water molecule is formed by two elements: two positive Hydrogen ions and one negative Oxygen ion.
The water molecule is held together by the
electromagnetic attraction between these ions. When
electricity is introduced to
water through two electrodes, a cathode (negative) and an anode (positive), these ions are attracted to the opposite charged electrode. Therefore the positively charged hydrogen ions will collect on the cathode and the negatively charged oxygen will collect on the anode.
EUREKA
-
Hydrogen is the most abundant element in the universe. With the "green-energy" craze and talk of powering our future oil-free economy on hydrogen, it has received much attention in the last few decades. Learning about this potential fuel of the future is important and
interesting, but not without snags, and these are for anyone to seek to
overcome.
ANIMATION
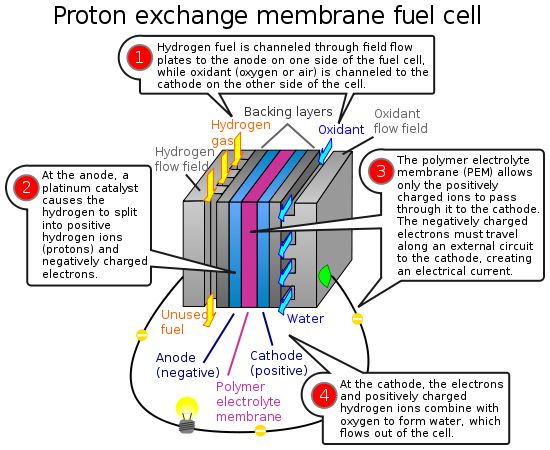
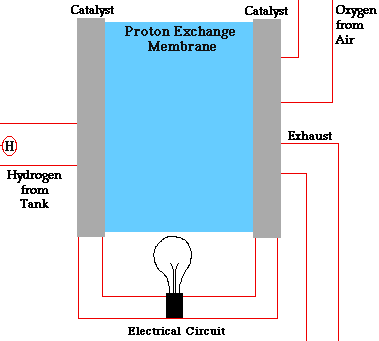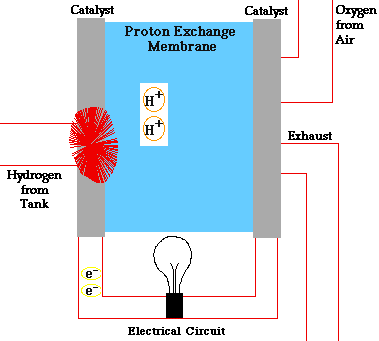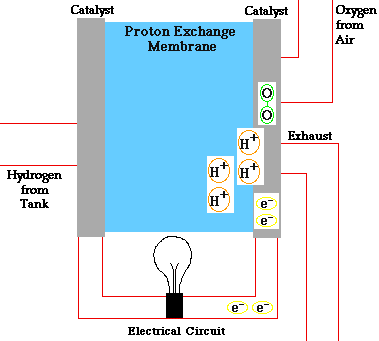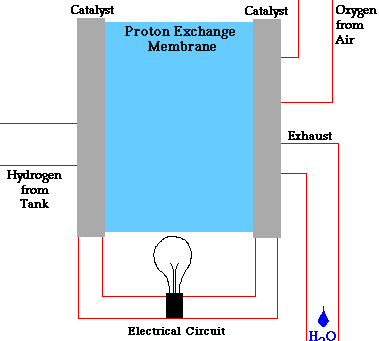
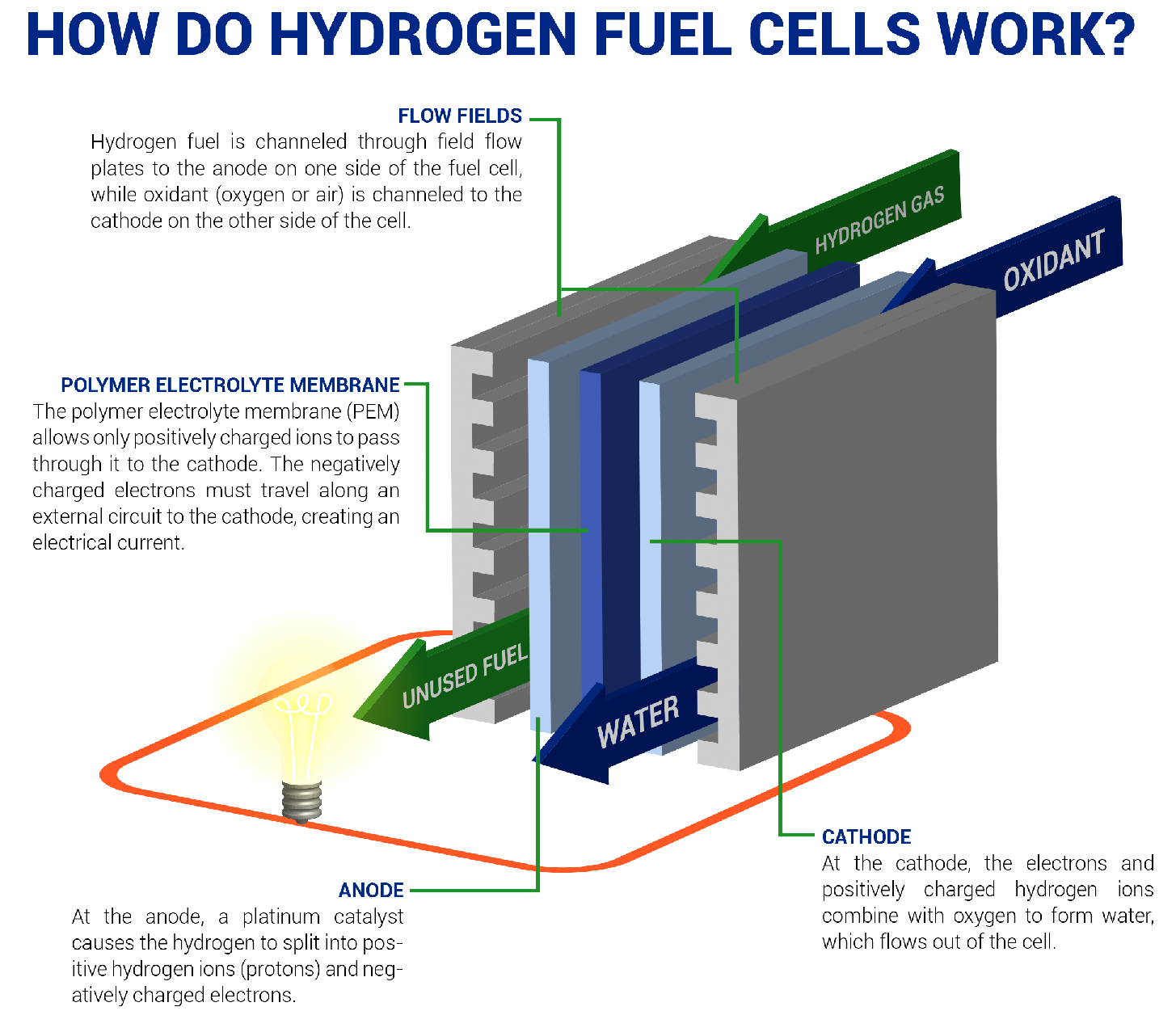
- A fuel cell converts the chemicals hydrogen and oxygen into water, and in the process it produces electricity.
A-Z
INDEX OF H2 POWERED FUEL CELL SHIPS
LINKS & REFERENCES
https://edu.rsc.org/feature/fuelling-the-future-solid-phase-hydrogen-storage/2020153.article https://www.cnbc.com/2019/02/21/musk-calls-hydrogen-fuel-cells-stupid-but-tech-may-threaten-tesla.html https://www.cnbc.com/2019/02/26/how-toyota-is-helping-japan-create-a-hydrogen-fueled-society.html https://www.ft.com/content/98080634-a1d6-11e7-8d56-98a09be71849 https://www.explainthatstuff.com/fuelcells.html
...
USA - In 2003, President Bush announced a program called the Hydrogen Fuel Initiative (HFI) during his State of the Union Address. This initiative, supported by legislation in the Energy Policy Act of 2005 (EPACT 2005) and the Advanced Energy Initiative of 2006, aims to develop hydrogen, fuel cell and infrastructure technologies to make fuel-cell vehicles practical and cost-effective by 2020. Obviously, the legislation did not work, or we'd seen hydrogen cars selling like hot cakes. Whereas, there are significant sales of battery electric cars.
The United States has dedicated more than two billion dollars to fuel cell research and development so far. Yet the basics principles of climate change is to find the best way to use less energy to achieve the same goal. Of course we have to explore all avenues before deciding on what works best. Thomas Edison found 1,000 ways not to make a light bulb before inventing his carbon filament version that succeeded. Joseph Swan in the UK filed a similar patent before the more famous US inventor. Keep at it chaps.
Please use our A-Z INDEX to navigate this site
AMMONIA - COMPRESSED GAS - ECONOMY - FUEL CELLS - FUSION - HYDRIDES - LIQUID GAS - METHANOL
|
||
|
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2022. Solar Studios, BN271RF, United Kingdom.
|